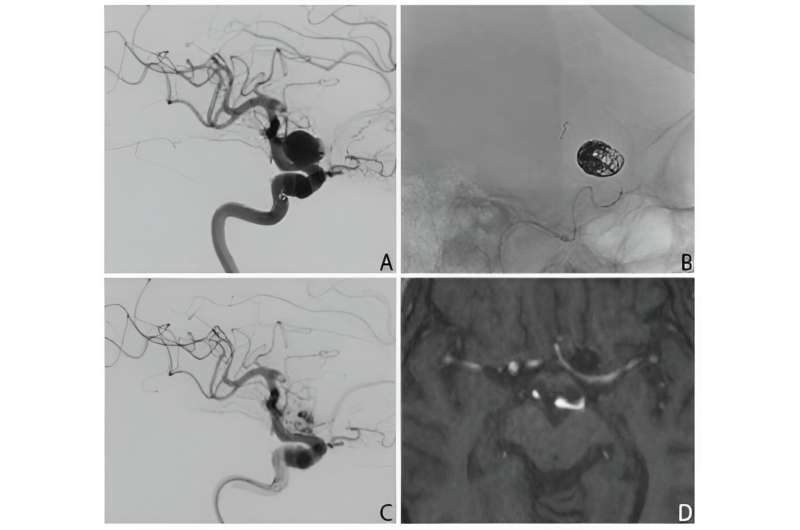
A new study, published in the Journal of Neurointerventional Surgery, has assessed the safety of updated Pipeline Vantage Embolization Devices (PEDV) used to stop blood flow into brain aneurysms as a part of treatment.
Using data from eight hospitals, King's researchers at the School of Biomedical Engineering & Imaging Sciences showed that a second updated version of the PEDV was acceptable in terms of stopping the blood flow into aneurysms effectively, being safe for patients with unruptured aneurysms, and technical difficulties related to placing the device into the artery had been overcome.
"It is always vital to assess new devices in the clinic to assess and compare how effective they are in treating the disease and how safe they are for the patients. Overall, these are acceptable outcomes in this pragmatic and non-industry-sponsored study," says Dr. Thomas C Booth, reader in neuroimaging.
Brain aneurysms are localized balloon-like weakness in the blood vessels that can rupture and be fatal. The PEDV is a "flow-diverting stent" conforming to the shape of an artery that covers the entrance to an aneurysm to prevent blood entering.
The device is a metal tubular structure that is inserted into the arteries using tiny plastic tubes (catheters) that have been navigated next to the brain aneurysm under image guidance.
The first version of the PEDV was a limited pre-market release that had technical difficulties when placing the device into the artery, which lead to its withdrawal from the market. The second version was released globally following modifications to address these technical difficulties. However, the second version had not been assessed in the clinic until now to confirm that the technical difficulties had been overcome and that it was safe and effective to use.
The study showed that the second version appeared easier to deploy compared to the first version, and for unruptured aneurysm treatment, the second version appeared to have a superior efficacy and similar safety profile to previous generation PEDVs.
More information: Thomas C Booth et al, Outcome study of the Pipeline Vantage Embolization Device (second version) in unruptured (and ruptured) aneurysms (PEDVU(R) study), Journal of NeuroInterventional Surgery (2023). DOI: 10.1136/jnis-2023-020754
Citation: Study confirms safety of new flow-diverting stent in the treatment of brain aneurysms (2023, December 12) retrieved 12 December 2023 from https://ift.tt/ADPMbvi
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
"flow" - Google News
December 12, 2023 at 11:06PM
https://ift.tt/8EIfodq
Study confirms safety of new flow-diverting stent in the treatment of brain aneurysms - Medical Xpress
"flow" - Google News
https://ift.tt/fe56XxP
https://ift.tt/JsIevBN
Bagikan Berita Ini














0 Response to "Study confirms safety of new flow-diverting stent in the treatment of brain aneurysms - Medical Xpress"
Post a Comment